Lotions and butters are some of the most popular bath and body products, but they’re also products that can raise the most questions. Questions such as “Do I need a preservative for my product?” “Which preservative do I use?” and “Are preservatives safe?” are some of the most common ones we see, and we hope this thorough guest post by formulation guru and fellow crafter Susan Barclay-Nichols will help clear up some confusion.
Susan is an expert in cosmetic chemistry, and in this post she gets down to the nitty-gritty of which preservatives work best in which products, their usage rates and their ingredients. It’s a one-stop-shop for everything preservative related! Read on to learn about the wide world of preservatives and how you can safely use them in your products.

A-M Note: If you’re concerned about parabens and the safety of preservatives in bath and body products, we’ve found these following resources to be helpful explanations: Parabens Puzzlement, More to the Parabens Puzzlement and Debate Over Parabens – Truth and Research. Borrowing from the blogs: “The FDA supports the use of Parabens as does the European Union….and under regimented testing by the cosmetics directive of the European Union they too, found no direct correlation of Parabens and cancer.”
Keep in mind, the type of preservative you choose is up to you, and I’m glad that there are effective options for everyone out there who wants to responsibly use a full spectrum preservative. All Bramble Berry preservatives have been approved for use in body products. Now onto the guide! – A.M.
_____________________________________________________________________________________________________________________________________________________________________
You’ll notice there are actually two areas of contamination in the product. The first is the great big green spot on the left side, but did you notice the brown-y orange streak to the right hand side of that on the side of the jug? Yep, there’s a little more contamination for you!

I made this lotion without preservatives to show you how quickly things can go off. I made this sixteen days ago. (It might have gone off earlier. This was the first chance I had to check it, to be honest!) I did all the things I generally do – I heated and held both phases, I made sure all my equipment and workshop was clean – but I left out the preservatives. I covered it tightly with Press & Seal in this jug after it had cooled to room temperature. It’s been cold in the workshop – below 10˚C – which is quite chilly considering a fridge should be kept at 0˚C to 4˚C, and room temperature is 18˚C to 22˚C. It is also quite damp back there as we’ve had a bunch of rain since Halloween – just about every day – which is normal for this time of year.
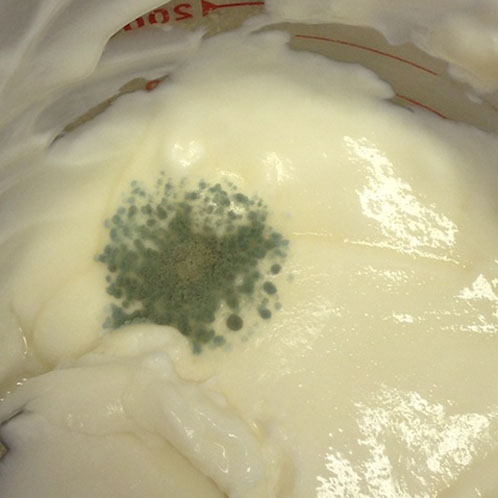
I have to point out that I get weary of hearing people saying they’re going to leave the preservative out of their products because “it’s just for me”. Do you want to smear THAT on your skin? Do you want to give that to someone you love? Please don’t. Products are contaminated a lot sooner than you think, and contamination is almost always there long before you see the green or orange bloom!
What could we have done to prevent such a tragic lotion contamination? Preservatives! Effective, broad spectrum preservatives suitable for a product that contains water, like a lotion.
What’s a broad spectrum preservative?
The ideal preservative will be a broad spectrum preservative, meaning it kills off bacteria, mold, yeast, and other fungi. The preservatives we buy are called synergistic preservatives, which are combinations of preservatives intended to eliminate all the various contaminants we could see in our products.

How do they work?
Preservatives kill the microorganisms in our products in a few different ways, but the primary way is to cause some kind of chemical disruption that leads to death. They leak their internal fluids, they can’t maintain pH, their cell walls break open, and so on.
Once the preservative has been used to attack a little beastie, it’s used up and can’t fight anything else. This is one of the reasons we need to preserve our products at a proper level and why we want to start with as little contamination as possible in our workshop, our ingredients, our equipment, and our packaging! Preservatives tend to live in the water phase of our products to fight any contamination that might show up in our creations because that’s where the beasties live. (They can migrate into the oil phase to fight beasties there, but most our problems are in the water phase!)
Which preservatives could we choose for our products? There are quite a few good and effective preservatives found at Bramble Berry, but you want to make sure you choose a broad spectrum one – or a combination of preservatives that will cover all the bases – suitable for your product.
For instance, if you want to make a sugar scrub that doesn’t contain water, you’ll want to pick something that is oil soluble, like Phenonip. If you’re creating a product that contains a lot of botanicals, you’ll want to consider Germaben II as it’s great for hard to preserve ingredients. If you want to make a lotion, you’ll want to choose something that works with water containing products or emulsions.
Let’s take a closer look at the preservatives you can find at Brambleberry!
Germaben II
INCI: Propylene Glycol, Diazolidinyl Urea, Methylparaben, and Propylparaben.

Germaben II is a liquid broad spectrum preservative that should be used at 0.5% to 1% in the cool down phase of your watery creations. The INCI is Propylene Glycol (56%), Diazolidinyl Urea (30%), Methylparaben (11%), and Propylparaben (3%), which means it is a formaldehyde releaser that contains parabens.
Because it contains three different preserving chemicals, it is suggested for use as a preservative for products that are hard to preserve, such as those containing a lot of botanical or proteins, like a toner or lotion with a lot of extracts and hydrosols. (I like it for things like strawberry extract that are very hard to preserve!) Although the manufacturer notes it can be added to your products at emulsification temperatures – up to 80˚C – it’s best used in your cool down phase at less than 60˚C.
Summary of Germaben II:
INCI: Propylene Glycol, Diazolidinyl Urea, Methylparaben, and Propylparaben.
Usage: 0.5% to 1% in the cool down phase of your creation
Water soluble, so it’s not suitable for anhydrous creations
pH range: 3.0 to 7.5
Optiphen
(INCI: Phenoxyethanol (and) Caprylyl Glycol)

Optiphen is a liquid preservative appropriate for fighting bacteria and yeast in our products (I can’t find any information on its efficacy against mould). Its INCI is Phenoxyethanol (and) Caprylyl Glycol. We know phenoxyethanol is a very effective against bacteria and yeast, but what about caprylyl glycol? It is paraben free and is not a formaldehyde donor.
Caprylyl glycol (aka 1,2-octanediol) is a good bacteriostatic ingredient (meaning it limits the growth of the bacteria but doesn’t kill it) and bactericide, but it isn’t very effective against yeast or fungi. Which means we really don’t have a fungal killer in this preservative.
Its suggested use is at 0.75% to 1.5% in the cool down phase of our product (best below 37.5˚C or 100˚F). It’s suitable for anhydrous products or things like sugar scrubs that do not contain water. It’s best in products with a pH of 6.0.
Some people report Optiphen can de-stabilize their emulsions, so it’s suggested to add this preservative at 45˚C to 55˚C (113˚F to 131˚F) and continue mixing until cool. It can thin out a cationic emulsion, but it will thicken as it cools, so don’t worry too much!
Summary of Optiphen:
INCI: Phenoxyethanol (and) Caprylyl Glycol
Usage: 0.75% to 1.5% in the cool down phase of your water based creations.
Suitable for anhydrous products
*Editor’s note: There is some debate over whether Optiphen can be used for water-based creations. You can read more about this on Susan’s blog. For water based creations, Optiphen ND is recommended.
No pH restrictions for this preservative.
Optiphen ND
INCI: Phenoxyethanol (and) Benzoic Acid (and) Dehydroacetic Acid

Optiphen ND is a liquid broad spectrum preservative with the INCI Phenoxyethanol (79% to 81%), Benzoic Acid (11.5% to 12.5%) Dehydroacetic Acid (7.7% to 8.5%). It is suitable for products containing water and can be added at any point in the product making process as it isn’t heat sensitive. It is best used with products with a pH under 6.0. It’s paraben free and isn’t a formaldehyde donor.
We know benzoic acid has moderate bactericidal activity and great fungicidal activity, and we know that phenoxyethanol has great bacteria and yeast killing abilities, but we don’t know anything about dehydroacetic acid. What does it do? Dehydroacetic acid is one of our organic acids. It has great fungicial properties but low bactericidal properties, so it’s a good addition to this mix to make it a broad spectrum preservative. Unfortunately, it tends to work very poorly when included at a pH of 5.0, and it can be inactivated easily by cationics, non-ionics, and proteins.
Summary of Optiphen ND:
INCI: Phenoxyethanol (and) Benzoic Acid (and) Dehydroacetic Acid.
Usage at 0.2% to 1.2% in any phase of your creation.
Suitable for creations containing water. Not suitable for anhydrous products.
May not be suitable for products that include cationic polymers or quaternary compounds.
Best used in products with a pH lower than 6.0.
Optiphen Plus
INCI: Phenoxyethanol (and) Caprylyl Glycol (and) Sorbic Acid

Optiphen Plus is a liquid broad spectrum preservative with an INCI of Phenoxyethanol (and) Caprylyl Glycol (and) Sorbic Acid. (It differs slightly from Optiphen with the inclusion of the sorbic acid.) As it isn’t heat sensitive, we can include it in our water-based creations at 80˚C or lower in the water phase of our process. It is not a formaldehyde donor.
We know phenoxyethanol is a good bacteria and yeast killer, while caprylyl glycol is a good bacteriostatic and bactericide, so why include sorbic acid? Sorbic acid is one of the organic acids, and it can be found paired with a calcium, magnesium, or sodium salt to help increase its solubility. It’s a good fungal, mould, and yeast inhibitor at pH 6.0 or less, and it’s an okay bactericide. It’s generally found in food stuffs at 0.01% to 0.1%. By combining these three preservatives, we have a great bacteria, yeast, mould, and fungal killing combination suitable for water-containing products. (I’m not sure if this version of Optiphen can curdle or destabilize your emulsions as I’ve never used it, so please report on your experiences in the comments!)
The one down side of using Optiphen Plus is the limited pH range of the product. It works best at pH 6.0 or lower, which means you will need to test your products to ensure they are in the right range. For instance, if you’re using decyl glucoside with a pH that can range from 7.5 to 11 as your primary surfactant, you’ll need to get that pH down substantially to play well with Optiphen Plus.
Summary of Optiphen Plus
INCI: Phenoxyethanol (and) Caprylyl Glycol (and) Sorbic Acid
Usage at 0.75% to 1.5% in the heated water phase of your product.
Suitable for products that contain water.
Good for products with a pH of 6.0 or under
Phenonip
INCI: Phenoxyethanol (and) Methylparaben (and) Ethylparaben (and) Butylparaben (and) Propylparaben (and) Isobutylparaben

Phenonip is a liquid, broad spectrum preservative. It can be used at 0.25% to 1.0% in all your creations, including anhydrous ones (those that do not contain water) because some of these parabens are oil soluble! With all these parabens, it is considered a very powerful preservative, so it’s suitable for those creations that might contain a ton of botanical or natural ingredients, like extracts or hydrosols. It’s paraben based and it is not a formaldehyde donor.
It’s suggested that we use Phenonip in the heated phase of our creations as it dissolves around 60˚C to 70˚C. If you are making a lotion, it’s suggested to divide the product up between the water and oil phases. If you want to use it in a cold product, heat up some propylene glycol or glycerin and add the Phenonip to that before adding it to your product. If you want to use it in a surfactant mix – say, a shampoo bar or body wash – then add it to the heated surfactant phase.
Phenonip is inactivated by some non-ionic ingredients, such as polysorbate 80 (at 5%, Phenonip is completely inactivated by polysorbate 80), and slightly by polysorbate 20 and 80 at 2.5%. It doesn’t do well with ceteareth-20 – it’s inactivated by 5% – but it is not affected by cetearyl alcohol. (I’ll have a post on preservatives and non-ionic ingredients shortly…)
Because Phenonip is oil soluble, it’s great for emulsified scrubs, oil based scrubs, lotion bars, scrub bars, hair care bars, and any other products that don’t contain water but might be exposed to it.
Summary of Phenonip :
INCI: Phenoxyethanol (and) Methylparaben (and) Ethylparaben (and) Butylparaben (and) Propylparaben (and) Isobutylparaben
Usage: In the heated phase of your product. Divide into the oil and water phases in lotions.
Suitable for all products, including anhydrous products.
Suitable for pH ranges of 3.0 to 8.0, so pretty much all of our products.
Preservatives in Action: Cold Weather Barrier Butter recipe
Let’s take a look at one of my favourite winter body butter recipes, and think about which preservatives might work well with it.
I’ve chosen to add cocoa butter at 5% to this product as it’s an approved barrier ingredient, which means it can help our skin prevent wind or cold chapping by creating an occlusive layer. I’ve chosen the calendula extract as it will help soothe irritated and sensitive skin. You could use another oil soluble extract in its place, such as chamomile or evening primrose extract, both of which work as great anti-inflammatories. And I’ve chosen rice bran oil as it has a lovely mix of linoleic and oleic acids, great for barrier repair and softening and moisturizing respectively, as well as a good amount of anti-inflammatory phytosterols.
Cold Weather Barrier Butter
Heated water phase:
43.5% water
10% aloe vera
3% glycerin
2% silk amino acids
2% DL-panthenol
Heated oil phase:
10% rice bran oil
10% avocado butter
5% calendula extract (oil soluble)
5% cocoa butter
7% BTMS-50 or Polawax
Cool down phase:
1% preservative
0.5% Vitamin E (anti-oxidant)
1% fragrance or essential oil
1. Weigh your water phase into a heat proof container and put into a double boiler.
1a. Weigh your total water phase on a scale – jug and all – so we can compensate for the lost water before mixing. And set some water in a separate container to heat. A pot with water on the stove or boiling up the kettle works well. You don’t need to boil it the whole time – bring it to boiling now and you’ll have some less-than-boiling water for step 3a.
2. Weigh your oil phase into a heat proof container and put into a double boiler.

3. Heat both phases to 158˚F and hold for 20 minutes. This is to kill any nasties that might be in any of our ingredients, as well ensuring both phases are the same temperature when we mix them together. (This is part of the emulsification process – the heating part of emulsification.)
3a. Remember how we measured the water phase in step 1a? Measure it again – zero your scale and measure the jug and all. Add enough of the warm water to get you to the total weight from step 1a.
4. When both phases reach 158˚F, pour one phase into the other and mix very well with a stick blender, hand mixer, or stand mixer. Mix periodically as the temperature drops.

5. When you reach 113˚F, add your cool down ingredients and mix very well.
6. Allow the lotion to come to room temperature before bottling so you don’t get condensation on the inside of the bottle or lid. I suggest using jars for this recipe as it’ll be quite thick.

7. Always label your bottle with the ingredients and date so you can replicate it or throw it away when the shelf life expires.

What preservatives would work with this recipe?
To determine this, we need to determine what this product is. It’s a lotion, which means it is an emulsion of oil and water. So we need to choose a preservative that works with water containing products and not something that works only with anhydrous (non-water containing) products. Our pH is slightly lower than 6 – around 5.8. We might be using a cationic or positively charged emulsifier like BTMS-50 or a non-ionic or neutrally charged emulsifier, so that’s important to remember. And we’re using harder-to-preserve botanical ingredients, like aloe vera, so we’ll want to use a preservative meant for that purpose or use one at maximum suggested usage levels.
Always take into consideration the container into which you’re putting your product when you’re done. Containers with disc caps, flip tops, and pumps are less likely to be contaminated compared to jars and containers with screw tops where products can be exposed to more air more frequently. If you’re using a jar for this lotion – something that’s a very good idea given how much butter we’ve used! – then you’ll want to use the maximum preservative in it. Which means we’d be using…
Germaben II – 1% in the cool down phase
Optiphen – 1.5% in the cool down phase
Optiphen ND – 1.2% in any phase. (Please don’t use this if you’re using BTMS-50 as your emulsifier as it doesn’t play well with cationic or positively charged emulsifiers.)
Optiphen Plus – 1.5% in the heated water phase
Phenonip – 0.5% in the heated water phase, 0.5% in the heated oil phase
When choosing which preservative to buy, you could work with just one that works for all products, like Phenonip, or get one for anhydrous (containing no water) products and one for water containing products. Use them at the maximum usage rate to start, then try them at slightly lower levels, if you wish, but no lower than the suggested minimum usage rate.
Regardless of which one or ones you choose, use them every time you make a product to avoid horrible and unsafe contamination issues like the ones I had in my unpreserved lotion! — Post by Susan Barclay-Nichols

If you’ve enjoyed this post by Susan, check out her e-books — they’re just as helpful and informative! From top left clockwise:
- Back to Basics (more than 50 recipes and explanations for making lotion bars, whipped butters, balms, oil based scrubs, bath melts, bath oils, oil based sprays, solid scrubs, and facial serums)
- Hair Care Products (everything you need to know about making and tweaking your own hair care recipes)
- Formulating Lotions & Creams (the 224 page e-book follow-up to the Lotion Making 101 e-Book)
- Lotion Making 101 (a 305 page e-book that includes many recipes for creating hand and body lotions, creams, body butters, and formulas to help you get started on formulating your own).





Which toiletries or cosmetics can I make only using phenoxyethanol (C8 H10 O2) as a preservative and what would their shelf life be in and out of the fridge? Research is a bit conflicting, some articles say it is a comprehensive preservative but other sites combine it with another preservative ( like yours). What are the temperature and PH restrictions for this preservative? I live in a country where everything has to be ordered from the UK, but I did manage to buy this preservative but now am at a loss of what to do with it. So far I’ve only made water/organic free recipes and liquid soap or Aloe Vera recipes which I keep in the freezer. Thank you for your invaluable information… everytime I google something… up pops your site.
Hi Jaslyn!
I’m not entirely sure! I do know phenoxyethanol is great for protecting against bacteria and yeast. However, our preservatives combine phenoxyethanol with other ingredients like Sorbic Acid, which helps prevent against yeast and other nasties.
My worry is that phenoxyethanol on its own may not protect against everything that can grow in lotion. I would recommend contacting the manufacturer of the preservative to find out more, including what the temperature and pH restrictions are. It may also be helpful to make a small test batch with it and keep it at room temperature. That way you can see if it protects against mold, yeast, fungi, etc in your products!
Also, we’ve had great luck with Optiphen ND for aloe vera recipes. Read more about the preservatives we carry here: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
You Wish Soap Supplies in the Netherlands carries Bramble Berry products, and may have some of those preservatives on hand! Read more about You Wish here: http://www.soapqueen.com/bramble-berry-news/soaping-world-resources-international-soapers/
-Kelsey with Bramble Berry
Your prompt reply is much appreciated. I realise I’m complicating the issue and should just order a comprehensive Preservative. I have to ask if you have in suppliers in Italy, otherwise will look at ordering from the Netherlands. For further on down the road… what ratio of sorbic acid to Phenoxyethanol do you advise?
I think comprehensive preservatives are the way to go!
We just have You Wish in the Netherlands, we don’t have a supplier in Italy. I hope you can get some products from them! As for the ratio of sorbic acid to phenoxyethanol, I’m not 100% sure. When you’re looking for a preservative, you want one that protects against a wide range of growth. We love Phenonip or Optiphen. Something similar to those would work well. 🙂
-Kelsey with Bramble Berry
for using Op ND how do you know if you have cationic polymers or quaternary compounds in your formulation? Is there a blog post on these two items or some where that is of quality yet that I can understand that you would recommend that is soaping/cosmetic related? Thank you thank you thank you all for your very very hard work and time. It shows and is deeply appreciated by many!~Lisa
Hi Lisa!
I have to admit, the science of how preservatives work is not something I am super familiar with! However, Susan, the author of this post, is great at explaining more about cationic polymers or quaternary compounds. You can learn more about those compounds in this blog: http://swiftcraftymonkey.blogspot.com/2010/10/cationic-polymers-polyquaterniums.html
-Kelsey with Bramble Berry
Thank you soo much for this and all of your content you publish. I tell everyone about yoursite and brambleberry and I’ve ordered from you. I was wondering I see that Op Plus is stable with calcium and magnesium. I was wondering if Phenonip is as well. Also how warm should you warm the vegetable glycerine when adding the preservative to it for Phenonip? and is their a minimum amount you should use to mix? I don’t really understand why it needs to udnergo the heated stage. Also I’m very interested in this preservative called Suttocide A. I was wondering if you could do a post explaining the use and instructions on it for the different products made in soaping world. I’m liking that one site says it works at a Ph of 3.5-12. I trust you and your company and would like to know what you think if your comfortable talking about this and maybe selling it if its a good thing. Thank you very much for your time and knowledge
Hi Lisa!
You are very welcome! Thanks so much for spreading the word about Bramble Berry and Soap Queen. 🙂
Optiphen Plus works great in water-based products, like toner and cleaning spray. Phenonip is a great all-purpose preservative that can be used in lotion, salt scrub and dusting powders. It can be used at .5-1% in your products.
Read more about preservatives here: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
The reason that Phenonip is heated slightly is to help it mix in well! It just needs to be slightly heated so the mixture is nice and fluid, allowing that preservative to mix in. Don’t make the mixture hotter than 200F, otherwise the Phenonip may start to degrade.
Thank you so much for your suggestion about Suttocide A! We haven’t worked with that product before, so it’s something we’ll consider for future posts.
Also, keep in mind soap doesn’t need a preservative. It has a pH level (9-10) that doesn’t allow mold to grow. 🙂
-Kelsey with Bramble Berry
This has been somewhat helpful but I’m kind of overwhelmed with this depth of info haha. The first batch of body butter I’ve made lasted for a long time with no noticeable mold growth. The ones afterwards contained honey and aloe vera gel which I’ve read contains water. With that being said I’m going back to the basics of using butter, oil, and essential oils. Would I still need to use a preservative and if so which would you recommend?
Hi Cazre!
I understand, preservatives can be a bit confusing!
As a general rule of thumb, preservatives need to be added when the product contains water (lotion), or will get water splashed into it during use (a scrub kept in the shower). We consider aloe vera liquid and gel similar to water, so we recommend a preservative in that case. If your aloe vera liquid already has a preservative, like ours does, you’ll still want to add one just in case. You can add it on the lower side though, around .5%.
Body butter doesn’t have water, and is typically stored in a dry place, so you don’t need a preservative. 🙂
If you’re selling a product and there is any chance it may get water in it, I would recommend a preservative to be on the safe side. Even with instructions to store the product in a dry place, it may still get water in it, which can lead to mold and bacteria growth.
This article has more information on preservatives and what types we carry: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
It’s a really helpful post. Also, if you’re ever unsure if you need a preservative in your product, don’t hesitate to ask us. 🙂
-Kelsey with Bramble Berry
Aloe vera liquid: https://www.brambleberry.com/Aloe-Vera-Liquid-P3704.aspx
Hi Guys!
Thanks so much for all the wonderful information and the knowledge you take the time to share with us. I frequently haunt the Soap Queen blog, but this is my first time commenting.
My main focus for a long time has been purely on perfumes (oils, solids, and in perfumer’s alcohol) so preservatives have never really been something on my radar, so to speak. That said, I have a question a product that I make. It’s an oil based serum for the face that contains nothing but a proprietary blend of exotic oils and essential oils that I’ve made and used for myself for years, but recently started offering it to my customers – who’ve gone gaga over it. I realize that since it’s an oil based product it TECHNICALLY doesn’t need a preservative, but it has occurred to me that since there always is the possibility that water could be introduced into the dropper bottle I package it in that perhaps I should be incorporating a preservative. Even though I always make sure that customers understand that it’s important to not introduce water into the bottle, it’s still a possibility. The last thing I want is to have nasties start growing in a customer’s bottle of serum.
What would you suggest as the best preservative option for a product like this, keeping an eye toward the “no formaldehyde, no parabens” crowd?
Thanks so much for your time!
Tina
Hi Tina!
That serum sounds amazing! So glad your customers are loving it. 🙂
I would recommend using Optiphen. It’s a paraben and formaldehyde free preservative that works great in oil-based products. We recommend using that at .5-1.5% in your products. Because it’s going on the face, I would recommend using .5%. Going higher than that can cause dryness. Using it at .5% will protect from mold and bacteria and prevent any dryness.
Read more in the Talk It Out Tuesday: Preservatives post: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
-Kelsey with Bramble Berry
May I ask for a face cream, and for a body cream, and lotion, what amount is best for those varying creams and what the shelf life would be? Thanks!
Hi there!
When it comes to the ingredients you add in your face and body creams, it’s definitely personal preference! For instance, if your face is on the oily side, you may consider an oil-free lotion, like this Oil Free Lavender Lotion: http://www.soapqueen.com/bath-and-body-tutorials/lotion/oil-free-lavender-face-lotion/
If your skin is on the dry side, this Lavender and Aloe Lotion would be a good choice: http://www.soapqueen.com/bath-and-body-tutorials/lotion/lavender-aloe-face-moisturizer/
You may want to use more lightweight oils in your facial lotion, like argan or sweet almond oil. That way it won’t feel too heavy on your skin. 🙂
For body lotion, you can use whatever oils you like! I love using avocado and sweet almond in my lotion. This Kissably Soft Lotion has avocado oil and feels amazing: http://www.soapqueen.com/bath-and-body-tutorials/lotion/kissably-soft-lotion/
Read more about how to create handmade lotion in this post: http://www.soapqueen.com/bath-and-body-tutorials/lotion/how-to-create-homemade-lotion-recipes/
As for the shelf life of the lotion, it depends on the oils added. For instance, if you add an oil with a short shelf life (like hazelnut oil), the lotion will last as long as that oil. So if your lotion contains oils with a shelf life of a year, that’s how long it will last. 🙂
Find a list of oil shelf lives here: http://www.soapqueen.com/bath-and-body-tutorials/tips-and-tricks/free-beginners-guide-to-soapmaking-common-soapmaking-oils/
-Kelsey with Bramble Berry
Hi Kelsey:
I’m very confused about the right preservative to add to my products. None of my products contain water. At this time I am not adding any preservatives and looking to add a safe preservative for my anhydrous products. Can you suggest a solution?
Regards,
Donna
Hi Donna!
Absolutely, I can definitely help! What are you wanting to add preservatives to?
-Kelsey with Bramble Berry
Hi guys, thank so much for this simple break down. To date, is there any paraben free preservative that can be used with BTMS ? Optiphen plus is definitely a curdler.
Hi Jane!
You’re welcome, so glad you like the post! We use Optiphen all the time in our lotions and scrubs with great results. Also, we use Optiphen ND in water-based products. Both are paraben and formaldehyde free and work great. 🙂
-Kelsey with Bramble Berry
Talk It Out Tuesday: Preservatives: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
Thanks Kelsey,
I’ll try those two and see if they curdle as well. Thank you! Just to be sure, they are broad enough in spectrum that I wont have to add another preservative e.g. for mold.
Hi again,
I thought I read that ND is easily inactivated by cationics? Wouldn’t the BTMS 25 or 50 then inactivate it?
Hi Jane!
We’ve found that Optiphen works better for oil based recipes, like lotion that has BTMS-50. Optiphen ND works better for water-based recipes like toners, that don’t contain BTMS-50. 🙂
-Kelsey with Bramble Berry
Hi Kelsey, am new to the whole skincare making thingy tho I have been doing some research since last year… so now I want to go for organic skincare range but very worried about the best preservative for each product cos I have a whole lot of recipe here for men and women skincare kits dunno if I send d recipes to you through mail , you can help in deciding which paraben free preservatives suits each of them… And also does bramble berry ship goods to Africa ? Thanks in advance
Hi Juliet!
Optiphen is a great option for you. It’s paraben and formaldehyde free and works in a wide variety of recipes, like lotion and scrubs.
Optiphen: https://www.brambleberry.com/Optiphen-P3682.aspx
We also have a great blog post that talks about each preservative we carry and what to use it in. You can read more in the Talk It Out Tuesday: Preservatives post: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
Also, we do ship to some countries in Africa! You can read more in our international shipping policies: https://www.brambleberry.com/International-Shipping-Policies.aspx
-Kelsey with Bramble Berry
I make sugar scrubs and there is so much debate about preservatives. My scrubs are not emulsified. I don’t like them. For the most part, Blogs, such as this one or swiftcrafty say to use a preservative suitable for oil based product. However. Over the last year, I have had much conversation with two chemist. One a microbiologist, 30 years with a well known world wide corporation and maker of all things house hold, from cleaners to food to cosmetics and soaps. The other a well known cosmetic chemist. They both say , that a scrub that is oil only can not be preserved. Basically the preservative gets locked into the oil. If and when water is introduced into the product via wet hands or the shower, the preservative is unable to get to the water to do it’s job. The only way to preserve a scrub is if it is emulsified, has a water phase and a preservative that works in both oil and water is used. I hear many people say that their preservative works, but I have not found anyone that has actually had them challenge tested to back up that claim. I would love to know I could use a preservative in my non-emulsified scrubs and know that it works and that I am not wasting my money. But I don’t. So I keep educating my customers of safe use and keeping the product dry.
Hi Darla!
Thanks for your thoughtful comment. While I have not heard these points before, they are certainly interesting. Personally, I have found that preservatives formulated for oil based products have kept my non-emulsified scrubs mold-free. I store mine in the shower, and have not had any issues, but I certainly understand your concerns. We instruct our readers and customers to use preservatives based on the instructions of the manufacturer of the preservative. From our customers feedback, they seem to work great. That being said, one of the great things about making your own products is being able to choose exactly what goes into them, and a preservative is a personal choice. It’s awesome that you make a point to educate your customers on how to correctly and safely use your product 🙂
-Amanda with Bramble berry
Hi. I am making a melt and pour soap with real aloe Vera gel in it (straight from the plant.) Would I need a preservative of any kind?
Thanks, Cadence.
Hi Cadence!
In our tests and recipes, adding a watery liquid like aloe vera gel can be difficult. It may not mix in to the soap very well, and can cause the soap to be watery.
We do have an Aloe Vera Melt and Pour you may like! It has 5% aloe vera gel and is very soothing. 🙂
Aloe Vera Melt and Pour: https://www.brambleberry.com/Aloe-Vera-Melt-And-Pour-P3185.aspx
-Kelsey with Bramble Berry
Hi, thank you for all this information, i can tell you put a lot of time on it.
I want to make a body scrub but i dont know what preservative to use, its an oil base preservative, and i dont want to use anything with barabens on it, any suggestion ? Thank you in advance for your time.
Hi Juliette!
Optiphen works great in scrubs, and is paraben and formaldehyde free! You can add that at .5-1.5% of your recipe. Also, make sure to add it when it’s 176F or below. 🙂
Optiphen: https://www.brambleberry.com/Optiphen-P3682.aspx
Talk It Out Tuesday: Preservatives: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
-Kelsey with Bramble Berry
Would Optiphen Plus also work in emulsified scrubs (no water added, just e-wax) and could I also use Optiphen Pkus in a water based body spray (water, aloe, polysorbate 20, glycerine, fragrance/essential oils)?
Hi Amanda!
Optiphen Plus works great in water-based products, so I think it would work great in the body spray recipe! It can be used at .75-1.5%.
For emulsified scrubs with no water, Optiphen or Phenonip would work. They work great for oil-based products. You can use them at 1%. 🙂
This post has more information on preservatives and how to use them: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
-Kelsey with Bramble Berry
I saw this product for sale http://www.luckyvitamin.com/p-30478-canus-goats-milk-lip-balm-015-oz. According to their ingredient list, it’s a lip balm that contains fresh goat’s milk but no preservative. How can they market this safely? I’ve been researching ways to incorporate goat’s milk into my body products, and I understood that anything with fresh goat’s milk would require a preservative (other than soap).
Hi Pam!
While I can’t speak for their products, I can tell you that lip balm with added goat milk does have a shorter shelf life. Because an actual milk is added to products, there is nothing that can be added to keep it from spoiling.
Milk naturally will stay fresh for a few weeks, then starts to go rancid. This will eventually happen in lip balms as well. You can keep the lip balm in the refrigerator to make it last longer, but there is nothing that can actually stop it from spoiling. Preservatives stop mold and bacteria, but do not extend the milk shelf life.
Hope that helps! If you have any other questions, please let me know. 🙂
-Kelsey with Bramble Berry
I just made your lovely Argan& Shea Lotion. It emulsified beautifully, initially. Then I added the green tea extract, lemongrass & rosemary EOs, Optiphen Plus and a few drops of color (that I typically use for lotion with success). Whisk, whisk, whisk at this point (too thick for the stick blender), and a few minutes later it began to separate slightly…more like a light curdle…boo hoo.
A similar thing happened once before with a face cream (also used BTMS-50), so I was starting to think it was the emulsifier. But (though I can’t recall for sure), I likely used Optiphen Plus for that recipe too….so now I’m thinking it’s maybe the preservative??
I put the cream in tubs, and it’s totally useable (for me and family) and feels wonderful once rubbed in. But I want to avoid this curdling phenomenon, especially when using nice ingredients like argan oil, shea butter and extracts. I’m almost out of the Optiphen Plus, think I’ll just stick with the Germaben II and Germal Plus for future makes (especially with BTMS-50)…in case that’s the offender.
Do you have any thoughts on what could cause this minor separation/curdling (almost makes the product almost “airy” and light). Ironically, the aforementioned face cream actually turned out to be my very favorite to date, but I probably wouldn’t give it to anyone since the texture isn’t exactly creamy like everyone expects.
Thanks for your thoughts!! Love your site….so helpful in so many aspects of “product” making. Started CP soaping recently, and I’m already ADDICTED :-)!!
Hi Tina!
I’m thinking it’s the Optiphen Plus! Optiphen Plus is typically used for water based products like room sprays and facial toners, so it may not work well in lotion, which has a lot of oil. However, Optiphen and Germaben work great in lotion. You can read more in the Talk It Out Tuesday: Preservatives post: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
I’m glad the lotion still feels great though! That way you and your family can enjoy the airy consistency. 🙂
-Kelsey with Bramble Berry
I am totally new at this and already have garnered the importance of a preservative. So if I am making a salt or sugar scrub and adding Phenonip, when would I add this, since there isn’t a heated phase?
Also I wanted to make a coffee sugar scrub but was concerned that I am just asking for it to go off with the coffee. Will the Phenonip keep it also and for how long?
Thank you of any advise.
Hi Leigh!
I would recommend adding the Phenonip to your oils before mixing the salt or sugar in. That way it is fully incorporated into your scrub. 🙂
Talk It Out Tuesday: Preservatives: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
Also, depending on the oils you used in your recipe, the scrub should have a shelf life of about a year. You can find out more about oil shelf lives in the Free Beginner’s Guide to Soapmaking: Common Soapmaking Oils post: http://www.soapqueen.com/bath-and-body-tutorials/tips-and-tricks/free-beginners-guide-to-soapmaking-common-soapmaking-oils/
-Kelsey with Bramble Berry
So I believe I read above that liquid soap doesn’t need a preservative? I do have hopes of doing liquid goat milk soap, along with lip balm, body butter and hard bar soap. In time, these would be for sale.
So how does one make liquid soap? I have read that one adds water to our soap bar? So wouldn’t this, then, need a preservative?
As mentioned in several posts above, I am worried about the health of our body when using preservatives. How do we know which ones are safe (been tested as such) for our body and why?
What about adding colors to any of our products? Which ones are safe to use? I work in a bakery and cringe every time I make a cake with die in it. I often wonder what its doing to me, when I have my hands green, red etc. LOL. And what is it doing for our customers? I want to learn more about safe colors.
Hi Peggy!
That is correct! Liquid soap has a pH that doesn’t allow mold to grow, so it doesn’t need a preservative.
Liquid soap involves combining oils and potassium hydroxide lye, then cooking your mixture for several hours. Then, you add water to dilute the base. We have an awesome step-by-step video that shows you how to make liquid soap. You can find that here: https://www.brambleberry.com/Liquid-Soapmaking-Online-Video-P4828.aspx
If you add goat milk to your soap mixture, it will saponify (or turn into soap) and the shelf life will be longer. However, if you dilute your mixture with goat milk, that shelf life will be a couple of weeks, longer if it’s stored in the fridge.
As for which preservatives are harmful, that’s a difficult question to answer! What is harmful to some may not be harmful for others.
All the preservatives we sell at Bramble Berry are skin safe. You may also like Optiphen, which is paraben and formaldehyde free.
Optiphen: https://www.brambleberry.com/Optiphen-P3682.aspx
I would recommend watching our Making Lotion From Scratch video. Anne-Marie talks at length about preservatives and everything else you include in your recipe and why.
Making Lotion From Scratch on Soap Queen TV: http://www.soapqueen.com/bath-and-body-tutorials/lotion/make-lotion-scratch-soap-queen-tv/
You can definitely add colors to your products, and all the ones sold at brambleberry.com are skin safe. I’ll include a post about that below. 🙂
Talk It Out Tuesday: Colorants: http://www.soapqueen.com/bath-and-body-tutorials/tips-and-tricks/talk-it-out-tuesday-colorants/
-Kelsey with Bramble Berry
I think to add water you just have to sterilize it or boil it.
The PH of all soap has to be high or if you add acid I believe it reverses saponifioncation process and makes a soap scum gunk. I’m wondering that gunk could grow some bacteria in it of it’s left out.
Hi Fia!
We recommend using distilled water in your lotion. Tap water, even boiled, can have microbes or bits of metal in it. That can cause mold and bacteria to grow.
I haven’t heard of citric acid reversing the saponification process! I do know adding citric acid to your soap helps lower the pH slightly, making that soap a bit more mild. We used it in our Sudsy Shampoo Bars: http://www.soapqueen.com/bath-and-body-tutorials/cold-process-soap/sudsy-shampoo-bars/
Soap residue is natural with handmade soap. We recommend using draining soap dishes for handmade soap so they stay more dry in between uses. Also, because soap has a pH doesn’t allow mold to grow, that soap residue shouldn’t form bacteria. I do find soap residue harder to clean after it dries, so it may be a good idea to wipe it frequently. 🙂
-Kelsey with Bramble Berry
Hello! I’ve just discovered your website and I am loving it! I especially love the spotlight on ingredients articles you do, I’ve learned so much!
I am hoping you can help me with a question I have. I plan on making myself some eye cream and I’d like to avoid using a preservative since my recipe does not contain any water and I’ll just be making a small batch for myself. However I’m not sure about the aloe vera gel I am planning to add. Even though it’s not water it it kind of water-y….should I be using a preservative if my recipe contains aloe vera gel? The aloe vera gel I bought is actually only “99.99” percent pure as they have added .001% of a preservative to the gel already. So I’m not worried about the aloe gel itself going bad, but will mixing it with the other ingredients (shea butter, almond oil, rose hip oil) shorten the shelf life?
Thanks so much for any insight you can share. I’ve tried reading through a bunch of comments on your various articles to see if my questions already been asked but couldn’t find anything so sorry if this is just a totally stupid question ;-\
Hi Mary!
So glad you like the blog!
That is a great question! We typically use a preservative when using aloe vera liquid, which is used as a water replacement. As for aloe vera gel, I’m not entirely sure! Because there is already a preservative in it, I would recommend contacting the manufacturer for more information. 🙂
-Kelsey with Bramble Berry
1. I just made a batch of body cream or lotion after the fact that I found out all about the perservatives. Can I add Optiphen to a finished batch?
2. I only make 8oz. lotion for me and don’t have a scale so when you say in the tutorial for lotion making, the perservative at 1% or .2oz that’s not 2 ounces so could you give it to me in measuring spoons.
Hi Rene!
I believe you can add that preservative to your cooled lotion. If it isn’t incorporating well, you can heat it in the microwave slightly to help it mix in. Just make sure your mixture is cooler than 176F, otherwise that Optiphen may not work properly.
Also, we prefer to measure by weight as it is more accurate. So, 1% of your mixture would be different than measuring spoons, as measuring spoons measure by volume. You can use our droppers though! They have mL marks on the spout for easy measuring. In that case, .2 oz would be about 6 mL. 🙂
-Kelsey with Bramble Berry
Optiphen: https://www.brambleberry.com/Optiphen-P3682.aspx
A Guide to Weight vs. Volume: http://www.soapqueen.com/bath-and-body-tutorials/tips-and-tricks/a-guide-to-weight-vs-volume/
Droppers: https://www.brambleberry.com/Droppers-With-Suction-Bulb-P3802.aspx
I have just started making cold process soaps and to test them out we made some body wash (soap + water) does this need a preservative since it is soap?
My next question is about a body butter that contains no water (just oils CO + Shea butter) would this need a preservative?
Hi Angie!
That’s a great question! Cold process and liquid soap has a pH level that doesn’t allow mold to grow, so it typically doesn’t need a preservative.
However, if you’re putting your bar soap into water, I’m not exactly sure! That’s not something we have tested. It may need a preservative because the water wasn’t mixed with the oils during the saponification process and allowed to turn to soap. Phenonip may be a good one to add. You can add that at .5-1% of your recipe.
Also, body butters don’t need a preservative. Recipes with water can grow mold or bacteria. Because butters don’t have water, they don’t need one! I’ll include a link with more information. 🙂
-Kelsey with Bramble Berry
Talk It Out Tuesday: Preservatives: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
Lots to think about! Thank you,all. I am leaning toward hot process soaps, lotion bars and bath soak formulations. (Lotion bars will probably come in contact with water at some point). What would you use for of any of these? I am really curious about hot process soaps because I don’t hear much about preservatives in connection with this method of soap making. Thank you
Marlene
Hi Marlene!
When making any kind of soap (cold process, hot process, melt and pour and rebatch) you do not need to use a preservative :). The pH level does not allow bacteria to grow. For bath soaks, these do not need a preservative either, because they generally do not contain water. Most crafters do not add preservative to lotion bars because they often do not contain oils either, but if you want to be extra safe you could certainly add one 🙂
-Amanda with Bramble Berry
I am glad to find the preservative info here. I was so surprised to recently have learned that so many of the preservatives are (or can be) deactivated by the use of emulsifying wax ( polysorbates)
!! Everyone I know uses the two together . This is Good stuff to learn!!
Hi Susan!
I’m so glad you have found this post helpful! 🙂
-Amanda with Bramble Berry
This is the best preservative article ive read all day lol! im just kinda confused by all the info out there. I bought Optiphen because I read it works in water base formulas so I figured I could add it to my bug spray and tanning oil recipes which are both at least 70% water and the rest is oil, extracts, and Plantamulse instead of poly 20. After reading this article im wondering if I should have bought Phenonip instead as it seems to be more broad spectrum than Optiphen. Im also working out a formula for a really bubbly bubble bath as well and it contains both oil and water, I also want to try my hand at sunscreen so im wondering what to use in both of those things as well, I though Optiphen would cover everything but I guess I didnt quite do enough research lol.
Hi Amanda!
All the information regarding preservatives can be a little confusing, I totally understand :). Which preservative is right for you is somewhat of a personal choice. Many people like to use Optiphen because it is paraben free and is not a formaldehyde donor. Phenonip is also a great choice because it’s broad spectrum, and can be used in a wide variety of projects. Phenonip would work for all of your project. There is some debate over if Optiphen can be used for water based creations. You can read more about this on Susan’s blog:
Optiphen Revisted:
http://swiftcraftymonkey.blogspot.ca/2011/11/optiphen-revisited.html
For water based creations, we recommend Optiphen ND. If you have any more questions, don’t hesitate to ask!
-Amanda with Bramble Berry
Hi Rhanda!
Because Optiphen Plus is a water soluble preservative, it would be great for a body spray which has a large water content. If you’re looking for a recipe, I would recommend taking a look at out White Ginger and Amber Spray Perfume tutorial. You can use this recipe, and simply switch out for whatever fragrance you would like. We did not use a preservative in this recipe because of the alcohol content, but you could add some if you’d like 🙂
White Ginger and Amber Spray Perfume:
http://www.soapqueen.com/bath-and-body-tutorials/white-ginger-and-amber-spray-perfume/
Regarding preservatives in your body wash, preservatives aren’t generally necessary in liquid soaps, but can be added if desired. The pH level in soap defends against mold and bacterial growth, but adding a little doesn’t hurt!
Yes, you can test the pH of your products using pH strips 🙂 They really come in handy when making so many things!
Ph Strips, 1 pack of 50:
https://www.brambleberry.com/pH-Strips-1-pack-of-50-P4434.aspx
I hope this helps!
-Amanda with Bramble Berry
Thank you so much! Yes, a huge help!
Would polysorbate 20 be essential to a body spray in order to incorporate your fragrance oils or essential oils? It shows it’s out of stock right now and I can’t wait for it! I plan to use glycerin in my body spray.
Regarding ph: I can’t help but worry my lotion (one of your recipes for basic body lotion) ph is not low enough for the optiphen plus to fully work. Can you tell me if lotions and creams are typically low ph? I made a batch of this lotion as a gift and am giving it away tomorrow and therefore cannot wait for strips in the mail to test! :/
Thank you ever so much for your time; it’s much appreciated!
Hi Rhanda!
You’re so welcome :). If you don’t use Polysorbate 20, your body spray will not incorporate, and the oils and water will separate. Of course you can do this but, but it’s not ideal.
Optiphen works between a pH of 2 – 8. Given that lotions are typically neutral (with a pH of between 6- 8), the Opitphen should work just fine! 🙂
Optiphen: https://www.brambleberry.com/Optiphen-P3682.aspx
Thanks a bunch! Hope the P20 comes in soon!
I’m pretty new here so I hope someone is still able to answer questions on an older post.
I have several questions regarding Optiphen Plus and would so appreciate any help. I recently purchased OP from Bramble Berry along with a ton of other lotion supplies and fragrances, and I’m wondering if I can use OP in body wash and body sprays along with my lotion? I read that the ph needs to be lower than 6 in order for the preservative to work. Would I need to test the ph level of all my lotions, body wash and sprays? And, if I do need to test them, would any old ph test strips work?
Also, do you have any advice/recipes/tips on making body spray? Thank you so much for any help.
Great article Susan. Preservatives is a subject close to my heart. I have written an article with reviews of common preservatives including those which are Ecocert “natural” approved –
http://makingskincare.com/preservatives/
As I comment in my post, as a general rule it’s always a good idea to mix up your preservatives, as they all have their strengths and weaknesses in what they kill, so a combo can give you a broad spectrum, and keep the overall levels of each down low, which helps with both stability and irritancy.
The finished pH of the product will have a major impact on the efficacy of the preservative. Bacteria thrive in the ph range of 5.5 to 8.5. Yeasts and filamentous fungi prefer the ph range 4-6. Use a good antifungal preservative at a low ph or a good antibacterial preservative at a high ph may provide a better broad spectrum preservation.
Reduce the amount of unbound water in your formulation – this will inhibit microbial growth. Lowering the water activity and water partitioning of a preservative prevents the migration of it away from the aqueous phase. This can be accomplished by increasing glycerin and other polyols above 5%; reducing the surface tension between the oil phase and water phase with functional siloxanes, particularly dimethicone polyethers and flurosilicones; and minimising sources of energy for microbial growth (e.g., carbohydrates, anionic surfactants, proteins, and natural gums).
Hi Jane!
Thanks so much for taking the time to leave a comment with such great info!
-Amanda with Bramble Berry
Is there a lab that tests PH level and also shelf life of products we create?
Ok, so how does a person know the PH of their finished product?
How do we know which preservatives are actually harmful to the body? This is what has actually held me back from making lotion etc. I raise dairy goats and am eager to learn to make goat milk products. I’ve got a friend who makes many products for sale. She said she prefers Liquid Germall Plus for the lotion. Comments?
Also say I’m going to make a body butter for sale. Keep in mind I’ve never made anything like this before. Say it contains Shea Butter, Extra Virgin Coconut oils. And I would use Essential Oils (far far more than just fragrances) from my trusted Essential Oil company). So no preservative is needed for something like this, if going to sell the product? I have read that after a little time, the butters (like Shea etc.) lose their potency. So how does a person know shelf life? Is there some place to test out this?
Hi Peggy!
We have pH test strips to check the levels of your products. I’ll include a link below. 🙂
pH Test Strips: https://www.brambleberry.com/pH-Strips-1-pack-of-50-P4434.aspx
As for which preservatives are harmful, that’s a difficult question to answer! What is harmful to some may not be harmful for others.
All the preservatives we sell at Bramble Berry are skin safe. You may also like Optiphen, which is paraben and formaldehyde free.
Optiphen: https://www.brambleberry.com/Optiphen-P3682.aspx
I would recommend watching our Making Lotion From Scratch video. Anne-Marie talks at length about preservatives and everything else you include in your recipe and why.
Making Lotion From Scratch on Soap Queen TV: http://www.soapqueen.com/bath-and-body-tutorials/lotion/make-lotion-scratch-soap-queen-tv/
As for liquid germall, we haven’t used that product, so I’m not entirely sure! We have great luck with the preservatives from brambleberry.com.
Also, that is correct – if the recipe doesn’t contain water, it doesn’t need a preservative. You can read more in the Talk It Out Tuesday: Preservatives post: http://www.soapqueen.com/bath-and-body-tutorials/lotion/talk-it-out-tuesday-preservatives/
As for the potency question, I’m still confused by that! Shea butter has a shelf life of about a year, so you’ll want to use if before then. You can find out about shelf lives in the Free Beginner’s Guide to Soapmaking: Common Soapmaking Oils post: http://www.soapqueen.com/bath-and-body-tutorials/tips-and-tricks/free-beginners-guide-to-soapmaking-common-soapmaking-oils/
-Kelsey with Bramble Berry
Although the marketing materials state that it is a broad spectrum preservative that works well with mold, the ingredients contained in Optiphen – phenoxyenthanol and caprylyl glycol – do not work with molds. We know phenoxyethanol is a very effective against bacteria and yeast, and we know that caprylyl glycol (aka 1,2-octanediol) is a good bacteriostatic ingredient (meaning it limits the growth of the bacteria but doesn’t kill it) and bactericide, but it isn’t very effective against yeast or fungi. Which means we really don’t have a fungal killer in this preservative. They can make a claim that it’s a broad spectrum preservative, but the chemistry doesn’t bear that out, unfortunately. (If you have other information, please send it along as I’d love to read more about this topic!)
The company’s material is a bit difficult to read at times because they’ll state something in one brochure, but leave it out in another. This is the case when it comes to whether we can use Optiphen in anhydrous products. I’m going to refer you to the post Optiphen revisited (http://swiftcraftymonkey.blogspot.ca/2011/11/optiphen-revisited.html) on my blog because it’s too long to recount here.
As for using it in anhydrous products, there is some debate about whether or not Optiphen Plus and Optiphen ND are suitable for anhydrous products. In their own marketing materials, ISP sometimes notes they are and, at other times, says nothing. This is the topic of much debate, and I don’t feel comfortable suggesting that someone use Optiphen ND or Optiphen Plus in anhydrous products. Phenoxyethanol is sparingly oil soluble, which means it can be dissolved in oil, but not enough that it could effectively protect your product.
Having said all of that, some people are comfortable using it in a product like an emulsified sugar scrub because the emulsified will help dissolve the phenoxyethanol in the product and keep any water that might enter the product from becoming contaminated. I suggest using it at the maximum level allowed and holding back a container to watch over time to make sure it keeps your product preserved. (Ideally, we’d all do challenge testing on our products, but that isn’t financially feasible!)
I really enjoyed the article–I’m making some sugar scrubs to give as gifts and I’m trying to figure out a preservative to use. I would be interested in what Susan’s response is to Marmar’s comment above and link regarding the discrepancy between Ashland’s information about Optiphen and the information she shared. Thanks!
Susan Barclay Nichols says:
December 14, 2013 at 7:13 am (Edit)
Although the marketing materials state that it is a broad spectrum preservative that works well with mold, the ingredients contained in Optiphen – phenoxyenthanol and caprylyl glycol – do not work with molds. We know phenoxyethanol is a very effective against bacteria and yeast, and we know that caprylyl glycol (aka 1,2-octanediol) is a good bacteriostatic ingredient (meaning it limits the growth of the bacteria but doesn’t kill it) and bactericide, but it isn’t very effective against yeast or fungi. Which means we really don’t have a fungal killer in this preservative. They can make a claim that it’s a broad spectrum preservative, but the chemistry doesn’t bear that out, unfortunately. (If you have other information, please send it along as I’d love to read more about this topic!)
The company’s material is a bit difficult to read at times because they’ll state something in one brochure, but leave it out in another. This is the case when it comes to whether we can use Optiphen in anhydrous products. I’m going to refer you to the post Optiphen revisited (http://swiftcraftymonkey.blogspot.ca/2011/11/optiphen-revisited.html) on my blog because it’s too long to recount here.
As for using it in anhydrous products, there is some debate about whether or not Optiphen Plus and Optiphen ND are suitable for anhydrous products. In their own marketing materials, ISP sometimes notes they are and, at other times, says nothing. This is the topic of much debate, and I don’t feel comfortable suggesting that someone use Optiphen ND or Optiphen Plus in anhydrous products. Phenoxyethanol is sparingly oil soluble, which means it can be dissolved in oil, but not enough that it could effectively protect your product.
Having said all of that, some people are comfortable using it in a product like an emulsified sugar scrub because the emulsified will help dissolve the phenoxyethanol in the product and keep any water that might enter the product from becoming contaminated. I suggest using it at the maximum level allowed and holding back a container to watch over time to make sure it keeps your product preserved. (Ideally, we’d all do challenge testing on our products, but that isn’t financially feasible!)
Thanks for posting that Anne-Marie. I definitely missed it the first time around. Thanks for the response Susan. Great article.
I love Susan’s Blog and Susan herself. She is a treasure and her experiments and supremely generous sharing of the findings along with extensive research makes her so awesome, and me very lucky indeed.
Thank you so much Brambleberry for shining a light on a great subject along with Susan because in the world of Bath and Body Chemistry she is Definitely a Keeper!
Hi Maryen!
I’m so glad to hear you enjoyed this post! We are so happy that Susan was able to share all her great knowledge with our soap community! We agree, she is a keeper for sure! 🙂
-Amanda with Bramble Berry
What is the url of Susan’s blog?
And I diddo all the great comments of knowing people. I want to learn to make these things.
Hi Peggy!
You can find Susan’s blog at http://swiftcraftymonkey.blogspot.com/.
Also, we have lots of fun tutorials on how to make lotion you may like. I’ll include a link below. 🙂
-Kelsey with Bramble Berry
Lotion: http://www.soapqueen.com/category/bath-and-body-tutorials/lotion/
This is great! I would LOVE a follow up blog post of what is NOT a preservative that some people will misconstrue as one. I know things like Vitamin E (a good antioxidant), GSE, and ROE (rosemary oleoresin extract) are not actual preservatives but I am constantly reading a lot of people who believe they are.
Hi Toni!
Glad you found this post enjoyable! Thanks for the suggestion, I will share it with my team! 🙂
-Amanda with Bramble Berry
So is this the Brambleberry blog? I was just going to email Brambleberry.
Hi Peggy!
This is the official blog of Bramble Berry! You can ask us questions here or email [email protected]. We’re happy to help. 🙂
-Kelsey with Bramble Berry
Hi Melissa. I have some posts in the chemistry section of my blog on what cationic, anionic, and non-ionic mean, but it sounds like you’re asking where to find out what charge each ingredient we use might be? As a general rule, surfactants that foam and lather are anionic or negatively charged; cationic polymers like polyquat 7, and cationic emulsifiers, like BTMS-50, will be positively charged; oils, butters, emulsifying waxes (unless otherwise specified), extracts, solubilizers, and just about everything we use is non-ionic or not charged. The best way to find out is to ask your supplier or get the data sheet or bulletin on the product.
The cream soap preservative discussion will have to wait for an expert soap-woman like Anne-Marie as I don’t make soap yet. (Although I thought cold process soap didn’t need a preservative?)
Hi,
I never tried making products before but I’m wondering if these preservatives can be added to baking soda & citric acid, that I keep separate until I want to use them both with cactus gel or seasons seed powered plus water.
I just don’t want the preservatives to have a bad chemical reaction with the endothermic reaction of baking soda & citric acid(aka volcano experiment).
I don’t know if the preservatives are even liquid which would set off th volcano effect with baking soda & citric acid. And if it’s it’s safe to use as bith even if it’s not liquid.
There are some oils or seeds that stay better if they’re not exposed to sunlight. Can skem of these preservatives make it possible for them to be exposed to sunlight & stay good?
Outstanding article. Many thanks to Susan and BrambleBerry for shedding more light on the mysteries of preservatives.
Hi Michelle!
So glad to provide you with more information about the products you might be using! 🙂
-Amanda with Bramble Berry
Optiphen preservatives are approved for use in all major markets, compatible with a variety of formulations and not based on paraben, formaldehyde or halogens. Effective against gram-positive and
gram-negative bacteria, yeast and mold, they offer excellent heat stability, work across a wide pH window and are easily solubilized in water.
Optiphen preservative
INCI Name: Phenoxyethanol (and) Caprylyl Glycol
Broad-spectrum activity against bacteria, yeast and mold additional fungicidal protection may be needed in difficult formulations
Effective over pH of 4 to 8
this information I got from Ashland Web page
http://www.ashland.com/products/optiphen-preservatives
the guest mentioned that she couldn’t find any information on its efficacy against mold . but according to the above info. it is effective against mold.
Also she said that “It is water soluble, so it’s not suitable for anhydrous products or things like sugar scrubs that do not contain water.” but actually according to lotioncrafter and the Herbarie it can be used to preserve anhydrous systems and emulsions.
Susan Barclay Nichols says:
December 14, 2013 at 7:13 am (Edit)
Although the marketing materials state that it is a broad spectrum preservative that works well with mold, the ingredients contained in Optiphen – phenoxyenthanol and caprylyl glycol – do not work with molds. We know phenoxyethanol is a very effective against bacteria and yeast, and we know that caprylyl glycol (aka 1,2-octanediol) is a good bacteriostatic ingredient (meaning it limits the growth of the bacteria but doesn’t kill it) and bactericide, but it isn’t very effective against yeast or fungi. Which means we really don’t have a fungal killer in this preservative. They can make a claim that it’s a broad spectrum preservative, but the chemistry doesn’t bear that out, unfortunately. (If you have other information, please send it along as I’d love to read more about this topic!)
The company’s material is a bit difficult to read at times because they’ll state something in one brochure, but leave it out in another. This is the case when it comes to whether we can use Optiphen in anhydrous products. I’m going to refer you to the post Optiphen revisited (http://swiftcraftymonkey.blogspot.ca/2011/11/optiphen-revisited.html) on my blog because it’s too long to recount here.
As for using it in anhydrous products, there is some debate about whether or not Optiphen Plus and Optiphen ND are suitable for anhydrous products. In their own marketing materials, ISP sometimes notes they are and, at other times, says nothing. This is the topic of much debate, and I don’t feel comfortable suggesting that someone use Optiphen ND or Optiphen Plus in anhydrous products. Phenoxyethanol is sparingly oil soluble, which means it can be dissolved in oil, but not enough that it could effectively protect your product.
Having said all of that, some people are comfortable using it in a product like an emulsified sugar scrub because the emulsified will help dissolve the phenoxyethanol in the product and keep any water that might enter the product from becoming contaminated. I suggest using it at the maximum level allowed and holding back a container to watch over time to make sure it keeps your product preserved. (Ideally, we’d all do challenge testing on our products, but that isn’t financially feasible!)
Hi Susan.
A great informative article- thank you! I have used preservatives in my water-based creations, but I have read that a preservative isn’t needed in a lotion bar because of its non-water based nature. I am now confused. Could you possibly clear this one up for me? Thank you!
Anna
You do not need a preservative in lotion bars since there is no water in there. I’ll let Susan chime in if she has a differing take on it though =)
She had mentioned using phenonip in lotion bars above, so that left me confused. Thank you for the response!
So exactly what is a lotion bar, and how does one use it?
So can a lotion bar be made with goat milk? As I mentioned earlier, I do raise diary goats, so have an abundance of goat milk ready for use. Can one make a lotion bar with goat milk? What would be a recipe?
I’m not necessarily in the spot to start creating my own recipes (formulations), as I’ve not made any of it yet. LOL. However, I’m eager to learn.
I also have high hopes of creating some products for resale.
Please, if anyone can tell me if there is a PH stick or something to use to test our PH levels? If so, what are they and where to get them? Also, how do we test out shelf life, without waiting for mega years to test them out? And do we see the old etc with naked eye? So how do we test out shelf life? How to package properly? Potency of the products used? I’m not worried at all about my essential oils, because I only use the ones from my trusted essential oil company. However, I do wonder about my other questions. Perhaps this could be another post, as well? RE: making products for sale?
But if anyone has thoughts on these above questions?
Hi Peggy!
A lotion bar is a solid product that is typically a combination of waxes, butters and oil. When you apply it, the heat from your skin starts to melt the bar and moisturize your skin. Because they don’t contain water, they don’t need a preservative. I’ll include a few recipes below.
Argan and Sandalwood Vanilla Lotion Bar: http://www.soapqueen.com/bath-and-body-tutorials/argan-sandwood-lotion-bar/
Lotion Bar Love: http://www.soapqueen.com/bath-and-body-tutorials/lotion/lotion-bar-love/
Usually, no liquids are added to lotion bars. You can add liquid to lotion recipes. We have tried out several lotion recipes with goat milk and haven’t found one we liked. If you find one that works for you, please let us know!
Also, If you’re using 100% goat milk in your recipe the lotion will last about 6 weeks, maybe a little longer in the fridge. However, if you’re only using 10% goat milk in your lotion recipe it will probably last about 6-9 months. This is including a preservative.
We sell pH test strips you may like!
pH Test Strips: https://www.brambleberry.com/pH-Strips-1-pack-of-50-P4434.aspx
Typically, lotion bars and lotions without goat milk have a shelf life of about a year. 🙂
Also, we have some great posts on packaging and selling your products. I’ll include them below.
How to Label Lotion: http://www.soapqueen.com/bath-and-body-tutorials/lotion/how-to-label-lotion/
Making Lotion From Scratch on Soap Queen TV: http://www.soapqueen.com/bath-and-body-tutorials/lotion/make-lotion-scratch-soap-queen-tv/
So You Want to Sell Your Soap (Part One): http://www.soapqueen.com/business/so-you-want-to-sell-your-soap-part-one/
Part Two: http://www.soapqueen.com/business/so-you-want-to-sell-your-soap-part-2/
Part Three: http://www.soapqueen.com/business/so-you-want-to-sell-your-soap-part-3/
You can also check out our Teach Soap Forum, where other crafters can talk about selling their products. Teachsoap.com/forum
As for the potency of the products, I’m a little confused by the question! Would you mind clarifying that for me? Thank you. 🙂
-Kelsey with Bramble Berry
How does Phenonip do with the Polysorbate 60 in E wax? I use E wax and Phenonip. Testing reveals no evidence of contamination in my lotions but since poly 80 inactivates Phenonip, I just wondered. . .
I was wondering the same thing. I recently switched to Phenonip from Germaben for my lotions. I made two test bathces one with the preservative and one without just to see what it would do to the texture, if anything. I was satified with my tests so I made a regular size bath (about 48oz) and after about a week they got a weird smell to them. It also got pockets of liquid throughout it. I’m not sure what happened. It’s my regular recipe, only difference is the preservative. My e-wax has poly-60 in it so that could be the problem.
From Susan to me via email:
I’ll refer you to the study in which they tested various non-ionic ingredients with Phenonip. On page 4, you’ll find the chart which notes that at 2.5% and 5% polysorbate 60, Phenonip was significantly deactivated. (http://dl.dropboxusercontent.com/u/1020026/inactivationofpreservativesbynonionics.pdf) I guess the question is how much polysorbate 60 are you using in your product? If you’re using 5% e-wax and polysorbate 60 makes up 50%, then you would have 2.5% polysorbate 60 in your product, which could inactivate the Phenonip. If you can get it tested, I’d recommend it.
The same study showed that 2.5% polysorbate 80 inactivates Phenonip significantly, but 5% doesn’t. Interesting result, eh?
I love Susan’s blog! She’s a wealth of information and her blog is full of fantastic entries to read (and search). I’m happy to see she is a guest blogger and is able to sell her books in your store.
Hi Mitch!
We are so glad you enjoyed this post, Susan sure knows her stuff doesn’t she? :). We love her blog as well!
-Amanda with Bramble Berry
Thank you for the great article. I use phenonip for my lotions but have been including optiphen into my oil only body butters and sugar scrubs just in case. I am surprised that it does not contain anything to fight mold. Im thinking now of switching to either optiphen plus or optiphen ND instead. I need something that works in a waterless formulas and is easy to use as far as temp and ph. I like that the optiphen family is paraben and formaldehyde free. What would be my best choice, considering these parameters. Thanks a ton!
Hi Amanda – Phenonip is an effective broad spectrum preservative. I use it in all the classes I teach. I don’t recommend switching. =) I’ll let Susan chime in though.
From Susan to me via email:
“There is some debate about whether or not Optiphen Plus and Optiphen ND are suitable for anhydrous products. In their own marketing materials, ISP sometimes notes they are and, at other times, says nothing. This is the topic of much debate, and I don’t feel comfortable suggesting that someone use Optiphen ND or Optiphen Plus in anhydrous products. Phenoxyethanol is sparingly oil soluble, which means it can be dissolved in oil, but not enough that it could effectively protect your product. I guess I’m wondering why you wouldn’t want to use Phenonip – which can be used in oil soluble creations – in your anhydrous creations? It’s a great preservative for non-water containing products!
A more complete post on this topic can be found here: http://swiftcraftymonkey.blogspot.ca/2011/11/optiphen-revisited.html
“
Thanks for the wonderful information. Where can we find more information on what is cationic, etc? And what would you use as a preservative in cream soap?
Hi Melissa – Susan answered this earlier but I’ll just copy it below since it didn’t thread properly:
Susan Barclay-Nichols says:
December 10, 2013 at 6:51 am (Edit)
Hi Melissa. I have some posts in the chemistry section of my blog on what cationic, anionic, and non-ionic mean, but it sounds like you’re asking where to find out what charge each ingredient we use might be? As a general rule, surfactants that foam and lather are anionic or negatively charged; cationic polymers like polyquat 7, and cationic emulsifiers, like BTMS-50, will be positively charged; oils, butters, emulsifying waxes (unless otherwise specified), extracts, solubilizers, and just about everything we use is non-ionic or not charged. The best way to find out is to ask your supplier or get the data sheet or bulletin on the product.
The cream soap preservative discussion will have to wait for an expert soap-woman like Anne-Marie as I don’t make soap yet. (Although I thought cold process soap didn’t need a preservative?)
Reply
As for cream soap, to be on the safe side, especially since it’s typically something people dip their hands into, I would use a preservative at 1%.
http://qualityassurance13.blogspot.com/2013/11/banning-of-five-parabens-by-european.html
Five parabens is not all parabens, and the ones they are banning are not very common in cosmetics.
http://www.chemsec.org/news/news-2013/july-september/1206-eu-to-ban-selected-parabens-in-cosmetics
Thank you for the excellent information!
Hi Debbie!
You’re so welcome, I’m glad you enjoyed this post!
-Amanda with Bramble Berry
Next year all paraben is banned from Europe 🙂
Interestingly enough, the ones they are banning are not commonly used in cosmetics and none of them are present in Germaben II, the most common paraben based product for preservatives.
Why not use a non-hexane processed Japanese Honeysuckle extract? The same quality used by Chinese medicine docs for hundreds of years. Ones that are not grown in Japan right now due to the radiation. It’s not a paraben, but while structurally close, it is not. However, it is a broad spectrum preservative.
Synthetic parabens are xenoestrogenic, and can disrupt hormones. It may not make a difference a time or two, but it will build up in the liver and fat after years of use. 99% of Americans have parabens in their urine. Sodium Methylparaben is a widely used preservative here in the States, and it has been banned in Europe. Some years ago now. We get exposed to more than enough synthetic and petroleum based products, why expose yourself to more, and one that is Aho easily absorbed and stored.
Hi FLMom, Susan emailed me this nuanced answer to post here:
Japanese Honeysuckle extract isn’t a broad spectrum preservative. It has some bactericidal abilities, but there is no evidence it can offer more than that. It contains a naturally occurring paraben called para-benzoic hydroxy acid, which behaves as the bactericide. You will have to add other preservatives to it to make it a broad spectrum preservative.
There is no difference between something derived synthetically and one derived naturally when we look at the molecule. For instance, we can create Vitamin C in the lab and take Vitamin C from a lemon and it’s the same molecule, they’re both Vitamin C molecules. If it didn’t look the same, it wouldn’t be the same molecule. So a Vitamin C molecule that doesn’t look identical to the one from the lemon isn’t a Vitamin C molecule, it’s something else. In this case, para-benzoic hydroxy acid is the same whether it is synthetically derived or comes from Japanese Honeysuckle.
Here’s a post from Personal Care Truth about Japanese Honeysuckle: http://personalcaretruth.com/2010/07/honeysuckle-extract-the-new-paraben-debate/
And another one: http://personalcaretruth.com/2010/07/honeysuckle-plant-extract/
There is a preservative that uses honeysuckle called NataPres (http://swiftcraftymonkey.blogspot.ca/2011/09/preservatives-natapres-ecocert.html), INCI Glycerin (and) Leuconostoc/Radish Root Ferment Filtrate (and) Lonicera Japonica (Honeysuckle) Flower Extract (and) Lonicera Caprifolium (Honeysuckle) Extract (and) Populus Tremuloides Bark Extract (and) Gluconolactone. This is not a broad spectrum preservative in most situations. You’ll have to add something for fungi and yeast. I’ve had a few people say on my blog that they still grew mold using it.
Data bulletin for NataPres: http://www.univar.com/es-MX/US/Industries/~/media/PDFs/US%20Corp%20Region%20PDFs/PC/Naturals/NataPres%20%20natural%20preservation%20from%20Univar.ashx