
Starting today, we are revisiting the basics of cold process soap with the Back to Basic series. Over the next two weeks, we will cover basic concepts and tutorials designed for the beginner in mind. If you have never made cold process soap before, now is the perfect time! The Back to Basics Soapmaking Kit includes all the ingredients you need to make all 4 projects. If you are a seasoned soaper, it’s always good for a little refresher course. For our first lesson, we are covering the most important part of soaping (other than having fun)…safety! In particular, we are chatting about lye safety, including how to safely mix and handle your lye solution. Pencils ready? Let’s get started!

In order to make soap, oils must emulsify with lye, which begins the saponification process. During this process it’s important to make safety a top priority. Sodium hydroxide lye is an inorganic compound commonly found in drain cleaners like Draino. Sodium hydroxide lye is highly caustic and has the potential to burn the skin. Like driving a car, sodium hydroxide is safe when handled properly. But because lye has the potential to be extremely dangerous, it’s important to take every safety precaution when making cold process soap.
Sodium hydroxide lye is available in various forms, such as flakes, pellets and powder. To make cold process soap, lye is introduced to a liquid like distilled water. The liquid dissolves the lye and creates a lye solution. Mixing water and lye creates an exothermic reaction that causes a dramatic temperature increase. Adding lye to room temperature water can cause the water to reach temperatures up to 200 ° F. The mixture also creates fumes, which should not be inhaled.


 Notice the streaks of oil in the photos below? These mixtures have not reached trace, because they are not thoroughly mixed. Some of the oils have not yet started saponification, and the mixture is not completely emulsified. These mixtures need more stirring and stick blending to reach trace. If the soap was poured into the mold at this point, the soap would not properly set up. There may also be pockets of unsaponified oil and lye in your soap, which may cause skin irritation.
Notice the streaks of oil in the photos below? These mixtures have not reached trace, because they are not thoroughly mixed. Some of the oils have not yet started saponification, and the mixture is not completely emulsified. These mixtures need more stirring and stick blending to reach trace. If the soap was poured into the mold at this point, the soap would not properly set up. There may also be pockets of unsaponified oil and lye in your soap, which may cause skin irritation.



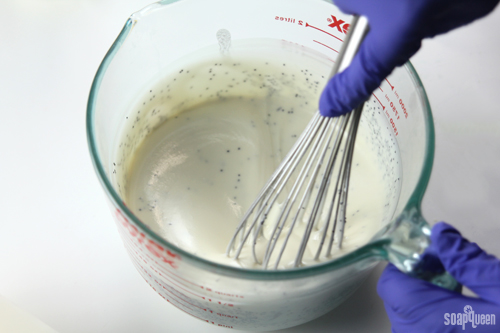 Adding poppy seeds into medium trace soap keeps them evenly suspended throughout the batter.
Adding poppy seeds into medium trace soap keeps them evenly suspended throughout the batter.
